TYLENOL® Safety Profile
TYLENOL® is an appropriate analgesic choice for many patients
TYLENOL® has an outstanding safety profile when used as directed. Studies have shown:
No evidence of acetaminophen toxicity at therapeutic doses for patients with stable chronic liver disease1
No evidence of liver dysfunction in a clinical trial with alcoholic patients2
Dose up to 4000 mg/day safely at a healthcare professional’s discretion for a wide range of patients*
*When used as directed.
†Maximum strength lidocaine without a prescription.
Use of Acetaminophen in Patients with Liver Disease
ALT relative to upper limit of reference range (ULRR) in patients with hepatic impairment
No statistically significant change from baseline.
Acetaminophen 4000 mg/day is well tolerated1
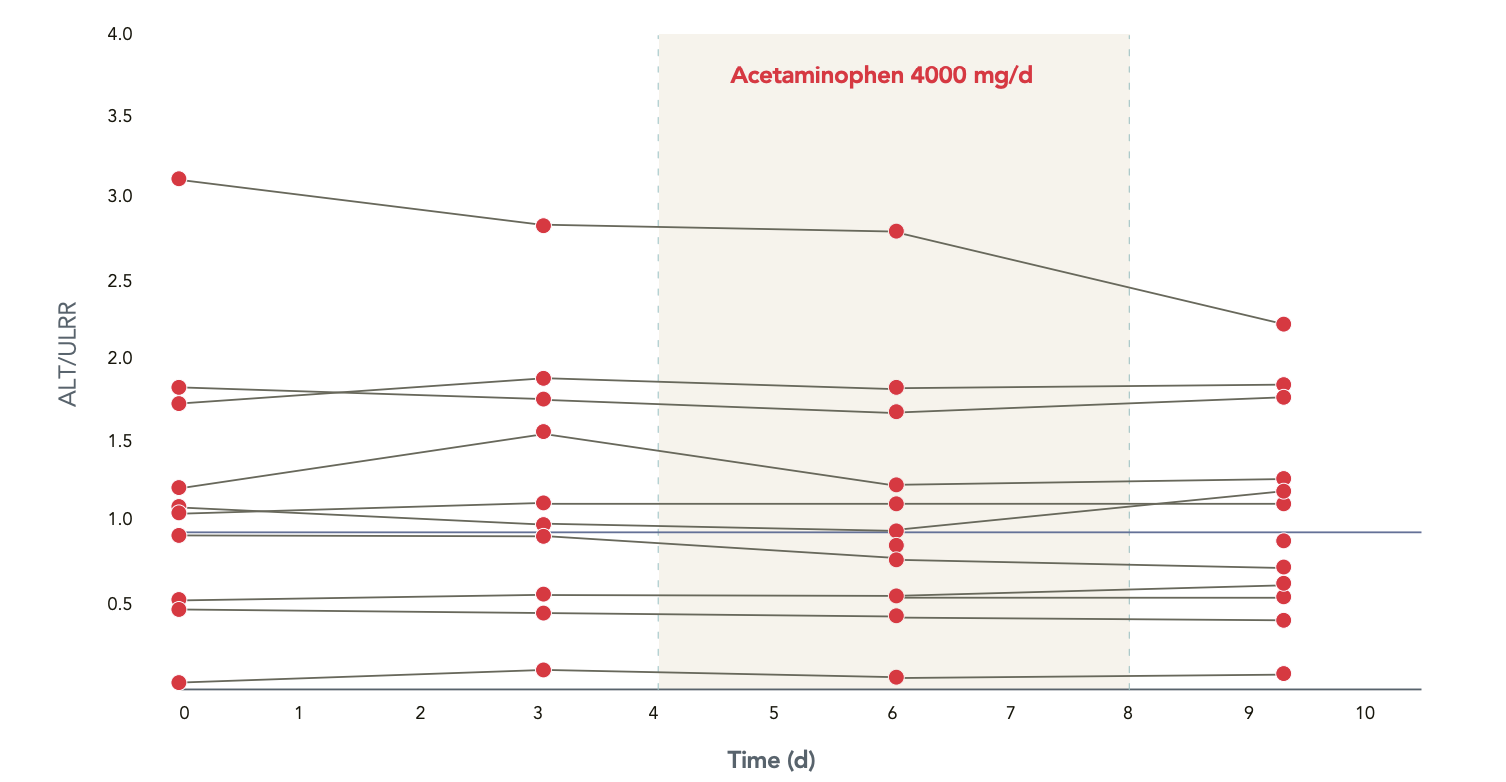
Study Design
10-day, open-label, parallel-group, in-clinic study
12 subjects with moderate hepatic impairment
13 healthy match-controlled adult subjects
Day 1: acetaminophen 1000 mg; Days 1 to 3: washout period; Days 4 to 8: acetaminophen 1000 mg every 6 hours
Measures: acetaminophen and metabolites in blood samples and pooled urine samples
*When used as directed.
†Maximum strength lidocaine without a prescription.
Use of Acetaminophen in Patients Who Consume Alcohol
Serum ALT measures in alcoholic patients treated with acetaminophen 4000 mg/day for 5 days—no statistically significant difference compared with placebo on any single day of the study2
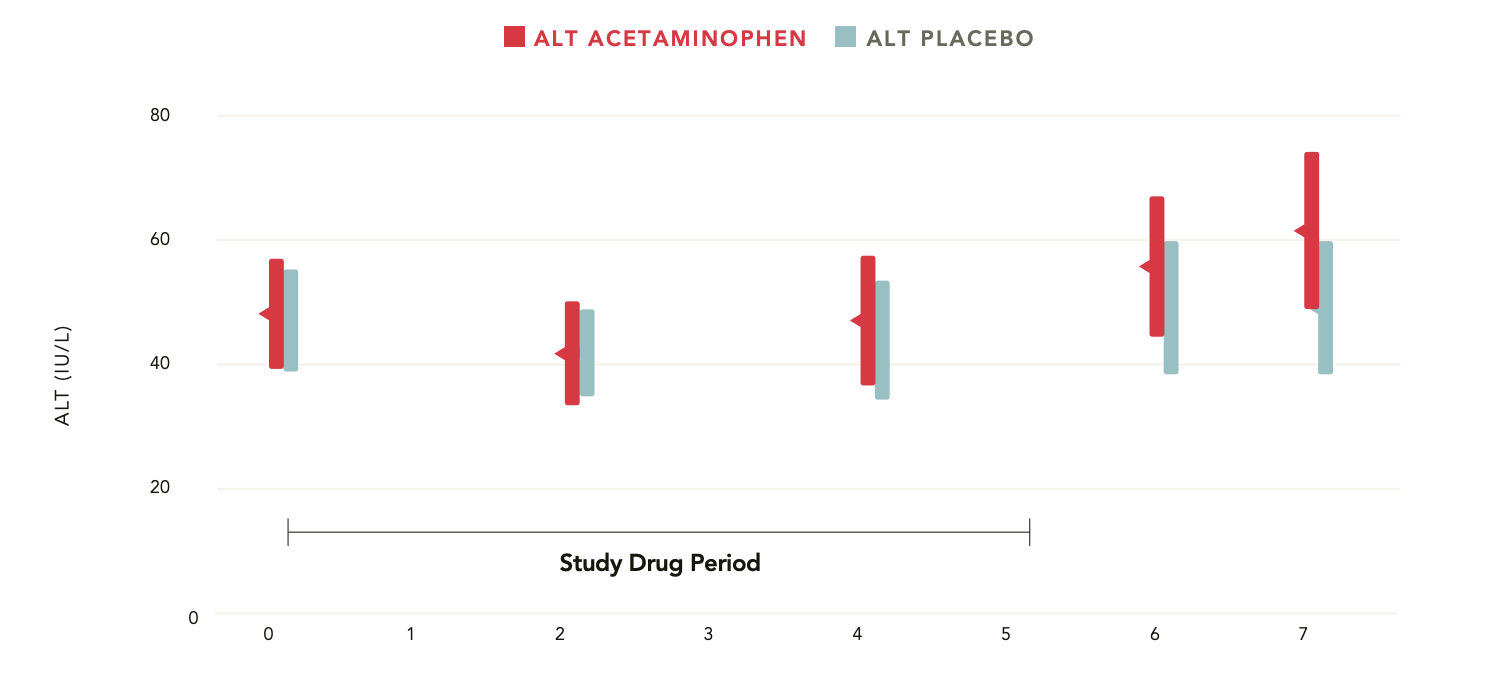
Study Design
Randomized, double-blind, placebo-controlled study
142 adult subjects who abuse alcohol
Groups: acetaminophen 4000 mg/day or placebo
5 days
Measures: serum alanine transaminase (ALT) and aspartate aminotransferase (AST), total bilirubin, et al
*When used as directed.
†Maximum strength lidocaine without a prescription.
Acetaminophen Label Liver Warnings
All TYLENOL® product labels have the following liver warnings:
Liver Warning: This product contains acetaminophen. Severe liver damage may occur if you take:
more than 4,000 mg of acetaminophen in 24 hours
with other drugs containing acetaminophen
3 or more alcoholic drinks every day while using this product
*When used as directed.
†Maximum strength lidocaine without a prescription.
References
1. Gelotte C, Temple AR, Zimmerman BA, Slattery JT. Acetaminophen biotransformation at single and repeat maximum doses is similar between adults with and without chronic liver disease. Poster presented at: 58th Annual Meeting of the American Association for the Study of Liver Disease; November 2007; Boston, MA.
2. Dart RC, Green JL, Kuffner EK, Heard K, Sproule B, Brands B. The effects of paracetamol (acetaminophen) on hepatic tests in patients who chronically abuse alcohol—a randomized study. Ailment Pharmacol Ther. 2010;32(3):478-486.
*The efficacy and safety of TYLENOL® at 4000 mg/day are well established.