Use these materials to guide proper dosage for TYLENOL® and MOTRIN® pediatric products
Jump to product:
- Infants’ TYLENOL® Oral Suspension
- Children’s TYLENOL® Oral Suspension
- Children’s TYLENOL® Chewables
- Children’s TYLENOL® Dissolve Packs
- Children’s TYLENOL® Cold + Cough + Runny Nose Oral Suspension
- Children’s TYLENOL® Cold + Cough + Sore Throat Oral Suspension
- Children’s TYLENOL® Cold + Flu Oral Suspension
- Infants’ MOTRIN® Concentrated Drops
- Children’s MOTRIN® Oral Suspension
- Children’s MOTRIN® Chewables
When pediatric patients have pain or fever, caregivers may turn to you with questions about dosing medicine safely—especially if it’s the first time. Infants’ and children's medicines are formulated to deliver the right amount of medicine to little patients. Dosage information for children under 2 years of age is available to healthcare professionals to share with caregivers.
Remind them to always read and follow the product label and use ONLY 1 medicine containing the same active ingredient at a time. Only the dosing device (dosing syringe or dosing cup) provided with the product should be used to measure the proper amount of medicine. Do NOT use more than one product containing acetaminophen at the same time.
| Pain & Fever Infants’ TYLENOL® Oral Suspension | |||
|---|---|---|---|
| DOSE: Repeat every 4 hours as needed. Do NOT give more than 5 doses in 24 hours. If possible, use weight to dose; otherwise, use age. |
|||
 ACTIVE INGREDIENT
Acetaminophen 160 mg (in each 5 mL) |
WEIGHT & AGE WEIGHT, AGE & DOSE | DOSE | |
| Under 2 years | |||
| 24-35 lbs2-3 years |
5 mL
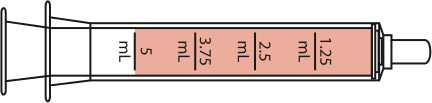 |
||
|
Available in:
|
|||
mL = milliliter
All Infants’ and Children’s TYLENOL® products have the same strength of acetaminophen: 160 mg (in each 5 mL, tablet, or powder).
| Pain & Fever Children’s TYLENOL® Oral Suspension | |||
|---|---|---|---|
| DOSE: Repeat every 4 hours as needed. Do NOT give more than 5 doses in 24 hours. If possible, use weight to dose; otherwise, use age. |
|||
 ACTIVE INGREDIENT
Acetaminophen 160 mg (in each 5 mL) |
WEIGHT WEIGHT, AGE & DOSE | AGE | DOSE |
| 24-35 lbs | 2-3 years |
5 mL
 |
|
| 36-47 lbs | 4-5 years |
7.5 mL
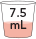 |
|
| 48-59 lbs | 6-8 years |
10 mL
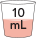 |
|
| 60-71 lbs | 9-10 years |
12.5 mL
 |
|
| 72-95 lbs | 11 years |
15 mL
 |
|
|
Available in:
|
|||
mL = milliliter
All Infants’ and Children’s TYLENOL® products have the same strength of acetaminophen: 160 mg (in each 5 mL, tablet, or powder).
| Pain & Fever Children’s TYLENOL® Chewables | |||
|---|---|---|---|
| DOSE: Repeat every 4 hours as needed. Do NOT give more than 5 doses in 24 hours. If possible, use weight to dose; otherwise, use age. |
|||
 ACTIVE INGREDIENT
Acetaminophen 160 mg (in each chewable tablet) |
WEIGHT WEIGHT, AGE & DOSE | AGE | DOSE |
| 24-35 lbs | 2-3 years |
1 tablet
 |
|
| 36-47 lbs | 4-5 years |
1 ½ tablets
 |
|
| 48-59 lbs | 6-8 years |
2 tablets
 |
|
| 60-71 lbs | 9-10 years |
2 ½ tablets
 |
|
| 72-95 lbs | 11 years |
3 tablets
 |
|
|
Available in:
|
|||
mL = milliliter
All Infants’ and Children’s TYLENOL® products have the same strength of acetaminophen: 160 mg (in each 5 mL, tablet, or powder).
Have questions about the recent dosage updates to Children’s TYLENOL® Chewables? Read common FAQs.
| Pain & Fever Children’s TYLENOL® Dissolve Packs | |||
|---|---|---|---|
| DOSE: Repeat every 4 hours as needed. Do NOT give more than 5 doses in 24 hours. If possible, use weight to dose; otherwise, use age. |
|||
 ACTIVE INGREDIENT
Acetaminophen 160 mg (in each powder) |
WEIGHT WEIGHT, AGE & DOSE | AGE | DOSE |
| Under 48 lbs | Under 6 years |
Do not use
|
|
| 48-59 lbs | 6-8 years |
2 powders
 |
|
| 60-71 lbs | 9-10 years |
2 powders
 |
|
| 72-95 lbs | 11 years |
3 powders
 |
|
|
Available in:
|
|||
mL = milliliter
All Infants’ and Children’s TYLENOL® products have the same strength of acetaminophen: 160 mg (in each 5 mL, tablet, or powder).
| Fever, Cold & Cough Children’s TYLENOL® Cold + Cough + Runny Nose Oral Suspension | |||
|---|---|---|---|
| DOSE: Repeat every 4 hours as needed. Do NOT give more than 5 doses in 24 hours. If possible, use weight to dose; otherwise, use age. |
|||
 ACTIVE INGREDIENTS
Acetaminophen 160 mg (in each 5 mL) Chlorpheniramine maleate 1 mg (in each 5 mL) Dextromethorphan HBr 5 mg (in each 5 mL) |
WEIGHT WEIGHT, AGE & DOSE* | AGE | DOSE* |
| Under 36 lbs | Under 4 years |
Do not use
|
|
| 36-47 lbs | 4-5 years |
Do not use unless
directed by a doctor |
|
| 48-95 lbs | 6-11 years |
10 mL
 |
|
|
Available in:
|
|||
mL = milliliter
All Infants’ and Children’s TYLENOL® products have the same strength of acetaminophen: 160 mg (in each 5 mL, tablet, or powder).
| Fever, Cold & Cough Children’s TYLENOL® Cold + Cough + Sore Throat Oral Suspension | |||
|---|---|---|---|
| DOSE: Repeat every 4 hours as needed. Do NOT give more than 5 doses in 24 hours. If possible, use weight to dose; otherwise, use age. |
|||
 ACTIVE INGREDIENTS
Acetaminophen 160 mg (in each 5 mL) Dextromethorphan HBr 5 mg (in each 5 mL) |
WEIGHT WEIGHT, AGE & DOSE* | AGE | DOSE* |
| Under 36 lbs | Under 4 years |
Do not use
|
|
| 36-47 lbs | 4-5 years |
5 mL
 |
|
| 48-95 lbs | 6-11 years |
10 mL
 |
|
|
Available in:
|
|||
mL = milliliter
All Infants’ and Children’s TYLENOL® products have the same strength of acetaminophen: 160 mg (in each 5 mL, tablet, or powder).
| Fever, Cold & Flu Children’s TYLENOL® Cold + Flu Oral Suspension | |||
|---|---|---|---|
| DOSE: Repeat every 4 hours as needed. Do NOT give more than 5 doses in 24 hours. If possible, use weight to dose; otherwise, use age. |
|||
 ACTIVE INGREDIENTS
Acetaminophen 160 mg (in each 5 mL) Chlorpheniramine maleate 1 mg (in each 5 mL) Dextromethorphan HBr 5 mg (in each 5 mL) Phenylephrine HCl 2.5 mg (in each 5 mL) |
WEIGHT WEIGHT, AGE & DOSE* | AGE | DOSE* |
| Under 36 lbs | Under 4 years |
Do not use
|
|
| 36-47 lbs | 4-5 years |
Do not use unless
directed by a doctor |
|
| 48-95 lbs | 6-11 years |
10 mL
 |
|
|
Available in:
|
|||
mL = milliliter
All Infants’ and Children’s TYLENOL® products have the same strength of acetaminophen: 160 mg (in each 5 mL, tablet, or powder).
| Pain & Fever Infants’ MOTRIN® Concentrated Drops | |||
|---|---|---|---|
| DOSE: Repeat every 6-8 hours as needed. Do NOT give more than 4 doses in 24 hours. If possible, use weight to dose; otherwise, use age. |
|||
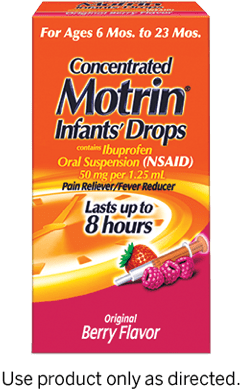 ACTIVE INGREDIENT
Ibuprofen (NSAID)* 50 mg (in each 1.25 mL) |
WEIGHT WEIGHT, AGE & DOSE | AGE | DOSE |
| 12-17 lbs | 6-11 months |
1.25 mL
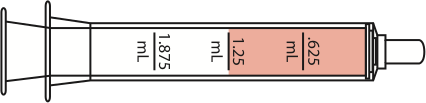 |
|
| 18-23 lbs | 12-23 months |
1.875 mL
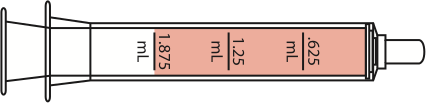 |
|
|
Available in:
|
|||
mL = milliliter
| Pain & Fever Children’s MOTRIN® Oral Suspension | |||
|---|---|---|---|
| DOSE: Repeat every 6-8 hours as needed. Do NOT give more than 4 doses in 24 hours. If possible, use weight to dose; otherwise, use age. |
|||
 ACTIVE INGREDIENT
Ibuprofen (NSAID)* 100 mg (in each 5 mL) |
WEIGHT WEIGHT, AGE & DOSE | AGE | DOSE |
| 24-35 lbs | 2-3 years |
5 mL
 |
|
| 36-47 lbs | 4-5 years |
7.5 mL
 |
|
| 48-59 lbs | 6-8 years |
10 mL
 |
|
| 60-71 lbs | 9-10 years |
12.5 mL
 |
|
| 72-95 lbs | 11 years |
15 mL
 |
|
|
Available in:
|
|||
mL = milliliter
| Pain & Fever Children’s MOTRIN® Chewables | |||
|---|---|---|---|
| DOSE: Chew or crush tablets completely before swallowing. Repeat every 6-8 hours as needed. Do NOT give more than 4 doses in 24 hours. If possible, use weight to dose; otherwise, use age. |
|||
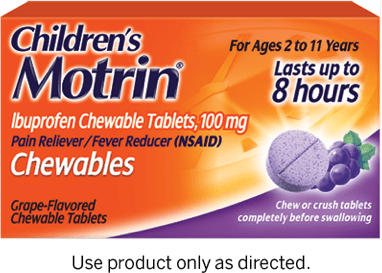 ACTIVE INGREDIENT
Ibuprofen (NSAID)* 100 mg (in each chewable tablet) |
WEIGHT WEIGHT, AGE & DOSE | AGE | DOSE |
| 24-35 lbs | 2-3 years |
1 tablet
 |
|
| 36-47 lbs | 4-5 years |
1 ½ tablets
 |
|
| 48-59 lbs | 6-8 years |
2 tablets
 |
|
| 60-71 lbs | 9-10 years |
2 ½ tablets
 |
|
| 72-95 lbs | 11 years |
3 tablets
 |
|
|
Available in:
|
|||
mL = milliliter
This is not a complete list.

 Grape
Grape Cherry
Cherry Dye-Free Cherry
Dye-Free Cherry Bubblegum
Bubblegum Strawberry
Strawberry Wild Berry
Wild Berry
Register
All Fields required, unless otherwise indicated
Personal Information
Step 1
Account Information
Step 2
Will be used as your user name
Address Information
Step 3
By submitting your information above, you agree that the information you provide will be governed by our site's Privacy Policy.